Abstract
Introduction
Patients (pts) with acute myeloid leukemia (AML) with poor-risk cytogenetics at diagnosis have inadequate responses to standard treatment. Pre-clinical and clinical data show that TP53mutation (mut) is associated with poor outcomes with venetoclax (Ven). However, the clinical impact of AML with poor-risk cytogenetics with and without a TP53 mut treated with a hypomethylating agent (HMA) and Ven is unknown. Herein, we evaluated the efficacy and safety of the Ven + azacitidine (Aza) or HMA combination in pts with treatment-naïve AML with poor-risk cytogenetics with TP53wt or TP53mut.
Methods
Treatment-naïve pts with AML unfit for intensive chemotherapy either due to age ≥75 years or co-morbidities were enrolled. Data were pooled from an ongoing phase 3 study (NCT02993523) comparing pts treated with Ven+Aza or placebo (Pbo)+Aza, and a phase 1b study (NCT02203773), in which pts were treated with Ven and an HMA, either Aza or decitabine (Dec). Ven 400 mg daily, Aza 75 mg/m 2 (days 1-7), or Dec 20 mg/m 2 (days 1-5), were administered over 28-day cycles. Cytogenetic risk was determined locally pre-treatment per NCCN (2016) criteria with the following abnormalities associated with poor outcomes: complex (≥3 clonal chromosomal abnormalities), monosomal karyotype, -5,5q, -7,7q, 11q23-non t(9;11) inv(3), t(3;3), t(6;9), and t(9;22). TP53mut status was assessed from pre-treatment BM aspirates at screening and analyzed centrally using MyAML panel assays (covering all coding and non-coding exons, limit of detection 1%; Invivoscribe). Pts without a result either due to an inconclusive test or missing specimen were excluded from the analysis. Response assessments were performed per modified International Working Group response criteria for AML.
Results
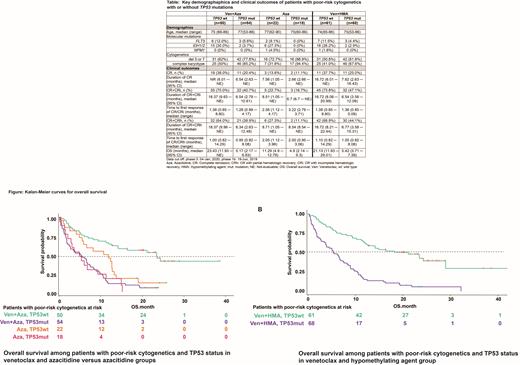
The pooled analysis included 546 pts; 353 and 145 pts in the Ven+Aza and Aza groups, respectively, with poor-risk cytogenetics observed in 127 (36%; TP53wt, 50; TP53mut, 54) and 56 (39%; TP53wt, 22; TP53mut, 18) pts. There were 401 pts in the Ven+HMA group with poor-risk cytogenetics observed in 152 (38%, TP53wt, 61; TP53mut, 68). The median variant allele frequency for TP53 was 42.9% (range 6.5 ─ 93.4) for Ven+Aza and 42.9% (3.5 ─ 93.7) for Aza. Key pt demographics are shown in the Table.
In pts with poor-risk cytogenetics +TP53wt who received Ven+Aza vs. Aza, the composite remission rates (CRc: complete remission [CR] + CR with incomplete hematologic remission [CRi]) were 70% vs. 23%, median duration of remission (DoR) was 18.37 (95% CI 9.63 ─ not evaluable [NE]) vs. 8.51 (1.05 ─ NE) months (mos), and median overall survival (OS) was 23.43 (95% CI 11.93 ─ NE) vs. 11.29 (4.90 ─ 12.78) mos (Figure A). Similar results were observed in pts treated with Ven+HMA: CRc rate was 74%, median DoR 16.72 (95% CI 8.08 ─ 20.99) mos, and median OS was 21.13 (95% CI 11.93 ─ 29.01) mos (Figure B).These poor-risk cytogenetics + TP53wt results were comparable to those of pts with intermediate-risk cytogenetics + TP53wt (Ven+Aza [n=166]: CRc 72%, DoR 21.91 mos, OS 19.15 mos; Ven+HMA [n=187]: CRc 72%, DoR 21.19 mos, OS 19.81 mos).
In pts with poor-risk cytogenetics + TP53mut who received Ven+Aza or Aza, CRc rates were 41% vs. 17%, median DoR was 6.54 (95% CI 2.79 ─ 10.61) vs. 6.7 (6.7 ─ NE) mos, and median OS was 5.17 (95% CI 2.17 ─ 6.83) vs. 4.9 (2.14 ─ 9.3) mos. In pts treated with Ven+HMA, the CRc rate was 47%, median DoR was 6.54 (95% CI 3.58 ─ 12.09) mos, and median OS was 5.42 (95% CI 3.71 ─ 7.39) mos.
In pts with poor-risk cytogenetics + TP53 wt, the common ≥ grade 3 (G3) adverse events (AEs) on Ven+Aza or Aza were thrombocytopenia (36%/36%), febrile neutropenia (32%/14%), anemia (26%/6%), neutropenia (24%/27%), and pneumonia (18%/23%). In pts with poor-risk cytogenetics + TP53 mut, the common ≥G3 AEs on Ven+Aza or Aza were also febrile neutropenia (43%/22%), thrombocytopenia (28%/28%), neutropenia (26%/17%), anemia (13%/32%), and pneumonia (26%/33%). In pts treated with Ven+HMA, the most common ≥G3 AEs in pts with poor-risk cytogenetics + TP53 wt or TP53 mut were similar.
Conclusion
Among pts with poor-risk cytogenetics +TP53wt, treatment with Ven+Aza or Ven+HMA was associated with higher remission rates and prolonged DoR and OS compared to pts treated with Aza alone. Outcomes were similar to intermediate-risk TP53wt Ven+Aza pts. In contrast, for similarly treated pts with poor-risk cytogenetics + TP53mut, improved response rates did not translate into improved DoR or OS.No new toxicities were noted.
Pollyea: Syndax: Honoraria; Takeda: Honoraria; Novartis: Consultancy, Honoraria; Foghorn: Honoraria; Genentech: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Jazz: Honoraria; Karyopharm: Consultancy, Honoraria; Kiadis: Honoraria; Amgen: Honoraria; Teva: Research Funding; Celgene: Honoraria; Syros: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Astellas: Honoraria; Aprea: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding. Pratz: Millenium: Research Funding; Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Agios: Consultancy; BMS: Consultancy, Honoraria; Cellgene: Consultancy, Honoraria; University of Pennsylvania: Current Employment; Abbvie: Consultancy, Honoraria, Research Funding. Wei: Novartis, Celgene, AbbVie, Servier, AstraZeneca, and Amgen: Research Funding; Novartis, Janssen, Amgen, Roche, Pfizer, Abbvie, Servier, BMS, Macrogenics, Agios, Gilead: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria. Pullarkat: Amgen, Dova, and Novartis: Consultancy, Honoraria; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees. Jonas: 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo Oncology, Pfizer, Pharmacyclics, Sigma Tau, Treadwell: Research Funding; AbbVie, BMS, Genentech, GlycoMimetics, Jazz, Pfizer, Takeda, Treadwell: Consultancy; AbbVie: Other: Travel reimbursement. Recher: BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; Janssen: Honoraria; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MaatPharma: Research Funding; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Babu: BMS: Consultancy, Honoraria, Other: Travel accommodations, expenses, Research Funding, Speakers Bureau; Novartis: Research Funding; Alexion: Consultancy, Honoraria, Other: Travel, accommodations, expenses, Speakers Bureau; Genentech/Roche: Other: Travel, accommodations, expenses, Research Funding; Janssen Oncology: Other: Travel, accommodations, expenses, Research Funding; Amgen, TG Therapeutics, AbbVie, Nektar, Sanofi, Argenx: Research Funding; Lily: Honoraria, Other: Travel, accommodations, expenses, Research Funding; Astra-Zeneca: Consultancy, Honoraria, Research Funding; Bayer, Castle Biosciences: Honoraria. Schuh: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlycoMimetics: Research Funding; Kite/Gilead: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dail: Genentech/Roche: Current Employment, Current equity holder in publicly-traded company. Sun: AbbVie: Current Employment. Potluri: AbbVie: Current Employment. Chyla: AbbVie: Current Employment, Current equity holder in publicly-traded company. DiNardo: GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; ImmuneOnc: Honoraria, Research Funding; Forma: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding.